By Anukriti Mathur and Si Ming Man
Food poisoning is a major health burden worldwide. Foodborne bacteria and their toxins can cause damage and massive inflammatory responses contributing to diarrhea and vomiting. An area of interest in our laboratory is to understand how the innate immune system recognize pathogens and their virulence factors. The innate immune system encodes hundreds of innate immune sensors that serve to detect pathogen-associated molecules and physiological changes induced by an infection. These innate immune sensors are highly efficient in triggering inflammation within the first few hours of infection to drive protective host responses.
Following a screen of clinically important human bacterial pathogens, we noticed that the foodborne bacterium Bacillus cereus secreted cytotoxic factors into the culture broth that can lead to rapid killing of macrophages. B. cereus is a ubiquitous Gram-positive bacterium which primarily causes food poisoning. It is an often-neglected pathogen which also causes debilitating extragastrointestinal diseases, including sepsis, soft tissue infection, blindness and meningitis.
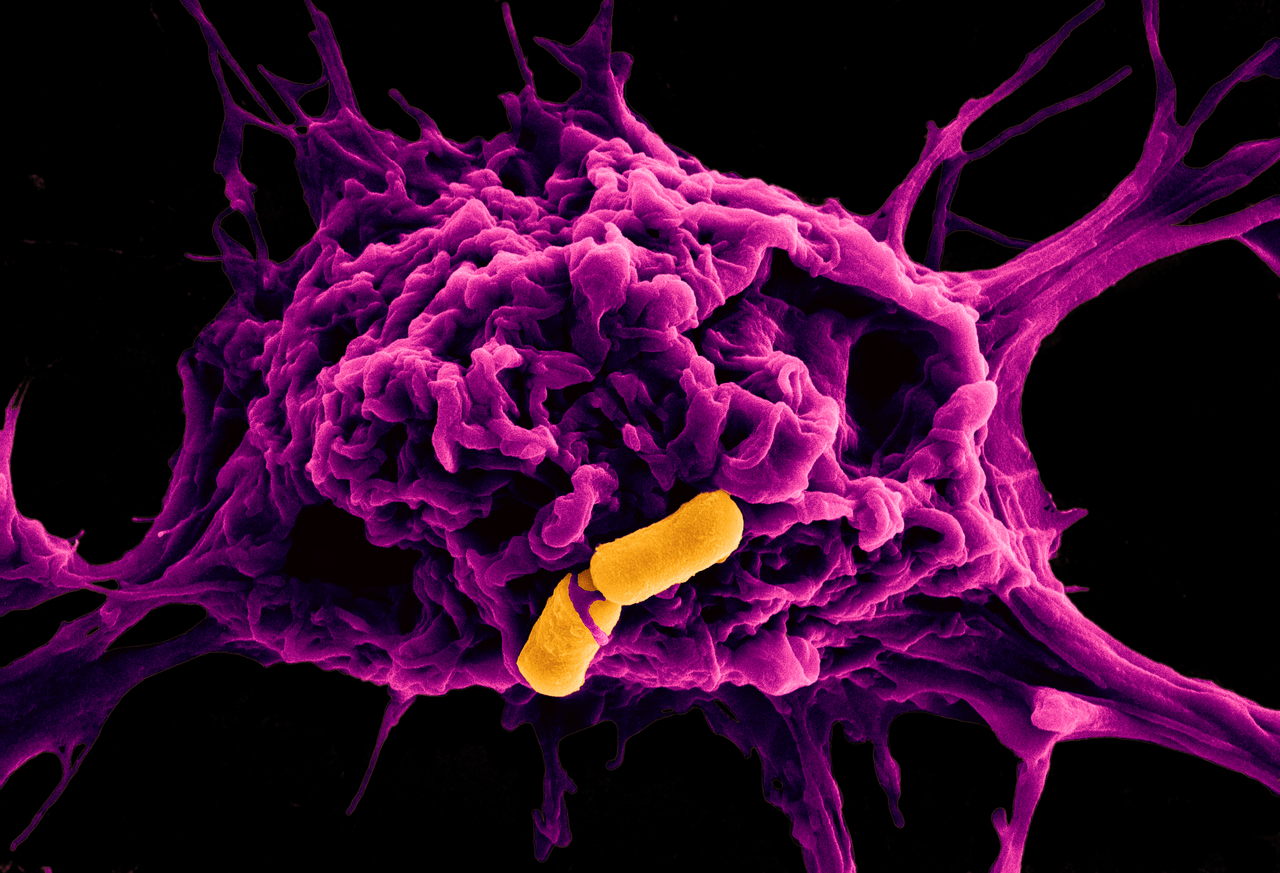
Death of the macrophages following stimulation with the culture broth of B. cereus was associated with the activation of the protease caspase-1 and secretion of the pro-inflammatory cytokines IL-1b and IL-18. These features are hallmarks of the inflammasome, a cytosolic macromolecular signaling hub regulating cell death and inflammation in the cell (Figure 1). These clues led us to identify the innate immune sensor driving activation of the inflammasome, which turned out to be NLRP3.
Previous studies have identified more than 10 different toxins produced by B. cereus. Following a series of experiments, we found that the secreted factor causing activation of the inflammasome and cell death was a heat-sensitive protein of 30-50 kDa in size. We also realized that the cytotoxic factor was in fact three different proteins, all of which are of 30-50 kDa in size. These three proteins assembled a multicomponent factor called haemolysin BL.
The biology of haemolysin BL is of particular interest given that the toxin is not pre-assembled in solution. We hypothesized that each component must be binding in a specific way on the plasma membrane of the host cell. Indeed, each subunit assembled one after the other, creating a pore on the cell membrane. The toxin pore allows potassium ions to escape the cell, generating a physiological change within the cell driving activation of the NLRP3 inflammasome, leading to cell death and inflammation. This ability of haemolysin BL to induce activation of NLRP3 mirrors that seen in several other pore-forming toxins, suggesting that NLRP3 might be a universal sensor of pore-forming toxins. Given that B. cereus produces multiple pore-forming toxins, it will be interesting to investigate why haemolysin BL, but not other pore-forming toxins from this bacterium, induces activation of NLRP3. We suspect that the size of the toxin pore and/or the binding capacity of each toxin might contribute to the selectivity of individual toxins.
How might we translate our findings to inform potential therapies? In a model of intraperitoneal infection, we observed that mice lacking the NLRP3 inflammasome do not readily succumb to the infection, whereas wild-type mice succumb rapidly within 20 hours. We suspect that overt inflammation might be driving immunopathology and septic shock in mice with a functional inflammasome response. These important data highlighted that therapeutic intervention to inhibit the NLRP3 inflammasome in sepsis-like infection might be beneficial. Indeed, administration of the NLRP3 inhibitory compound, MCC950, completely prevented the disease induced by B. cereus infection in mice.
Collectively, our study revealed NLRP3 as the cytosolic immune sensor of the B. cereus toxin haemolysin BL. Our study further supports the therapeutic relevance of targeting the inflammasome pathway in the treatment of infectious diseases.

Figure 1: A battle between a macrophage (magenta) and two rod-shaped B. cereus (orange).




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in